Abstract
Introduction: Smoldering multiple myeloma (SMM) prognostication models routinely used in clinical practice were developed for use at diagnosis. However, retrospective studies have shown that in SMM patients the risk of progression to multiple myeloma (MM) decreases over time. Therefore, this study assessed whether the Mayo 2018 and IMWG 2020 scores could be used dynamically to risk stratify patients post-diagnosis, and whether they could identify SMM patients with evolving disease.
Methods: We retrospectively studied 704 SMM patients diagnosed between January 2000 to January 2020. Patients with a baseline FLCr ≥100 and involved FLC ≥10 mg/dL or baseline bone marrow plasma cells ≥60% were excluded. We collected serial laboratory data and available pathology data and re-applied the Mayo 2018 and IMWG 2020 SMM risk stratification models at annual landmark timepoints up to 5 years post SMM diagnosis. Survival analyses were performed using the Kaplan-Meier method. Time to progression (TTP) was defined as time from diagnosis or landmark timepoint to treatment initiation for MM or systemic AL amyloidosis. Cox proportional hazards models were used to estimate hazard ratios. A two-sided p-value <0.05 was considered statistically significant.
Results: At SMM diagnosis, 271 (38%) patients were low risk, 228 (32%) were intermediate risk, and 205 (29%) were high risk per the Mayo 2018 score. Applying the IMWG 2020 score at diagnosis, 90 (34%) of patients were low risk, 111 (42%) were low-intermediate risk, 54 (21%) were intermediate risk, and 9 (3%) were high risk. The Mayo 2018 and IMWG 2020 risk scores was re-assessed annually post-diagnosis in patients without progression (respective sample sizes: n=430 and n=197 at year 1 landmark, n=326 and n=143 at year 2 landmark, n=260 and n=106 at year 3 landmark, n=203 and n=73 patients at year 4 landmark).
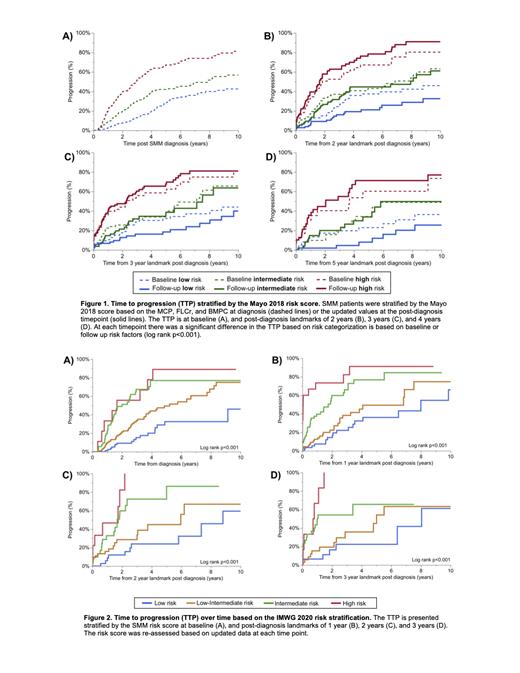
The Mayo 2018 and IMWG 2020 models reliably stratified patients based on progression risk post diagnosis (as shown in Figure 1 and Figure 2, respectively). As shown in Figure 1, if only diagnostic values were used in risk categorization with the Mayo 2018 model, the TTP between risk categories became less distinct over time. However, when follow-up laboratory values were used to re-stratify patients, the TTP between risk categories was more consistent over time. The respective 2-year progression risk in Mayo 2018 high-risk versus IMWG 2020 intermediate-high risk patients was 51% versus 62% at the 1-year landmark, 60% versus 65% at the 2-year landmark, 47% versus 62% at the 3-year landmark, and 47% versus 45% at the 4-year landmark.
To assess the prognostic significance of an increase in SMM risk category, we stratified patients based on whether the SMM risk category at follow-up had increased versus was stable or decreased compared to the baseline SMM risk stratification. We found that patients evolving to a risk category during follow up consistently had an increased risk of progression compared to patients with a stable/decreased risk categorization using both the Mayo 2018 (HR 3.34, 95% CI 2.04-5.48, p<0.001 at 2-year landmark; HR 3.41, 95% CI 2.05-5.68, p<0.001 at 3-year landmark; HR 2.69, 95% CI 1.34-5.38, p=0.005 at 4-year landmark) and IMWG 2020 (HR 2.32, 95% CI 1.42-3.77, p<0.001 at 1-year landmark; HR 3.10, 95% CI 1.69-5.70, p<0.001 at 2-year landmark; HR 2.50, 95% CI 1.30-4.78, p=0.004 at 3-year landmark; HR 2.26, 95% CI 0.91-5.60, p=0.072 at 4-year landmark) risk models. However, patients categorized as Mayo 2018 high-risk at follow-up had a similar risk of progression regardless of whether the baseline risk categorization was low-intermediate versus high-risk (HR 0.87, 95% CI 0.51-1.48, p=0.606 at 2-year landmark; HR 1.01, 95% CI 0.59-1.73, p=0.972 at 3-year landmark; HR 0.75, 95% CI 0.34-1.65, p=0.467 at 4-year landmark).
Conclusions: Our findings support the use of the Mayo 2018 and IMWG 2020 scores post-diagnosis. We showed that patients migrating to a higher risk category have an increased risk of progression. This suggests that if patients evolve to a high-risk score during follow-up, they should be considered for an early intervention treatment approach.
Kapoor: Sanofi: Consultancy; BeiGene: Consultancy; Pharmacyclics: Consultancy; Karyopharm: Consultancy; Cellectar: Consultancy; Amgen: Research Funding; Ichnos Sciences: Research Funding; Regeneron Pharmaceuticals: Research Funding; Glaxo SmithKline: Research Funding; Karyopharm: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; AbbVie: Research Funding. Dispenzieri: Pfizer: Research Funding; Janssen: Consultancy, Research Funding; Alnylam: Research Funding; Takeda: Research Funding; Oncopeptides: Consultancy; Sorrento Therapeutics: Consultancy. Gertz: Aurora Biopharma: Other: Stock option; Akcea Therapeutics, Ambry Genetics, Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Karyopharm Therapeutics, Pfizer Inc (to Institution), Sanofi Genzyme: Honoraria; AbbVie Inc, Celgene Corporation: Other: Data Safetly & Monitoring; Akcea Therapeutics, Alnylam Pharmaceuticals Inc, Prothena: Consultancy; Ionis Pharmaceuticals: Other: Advisory Board. Dingli: Janssen: Consultancy; Sanofi: Consultancy; Apellis: Consultancy; Novartis: Research Funding; Alexion: Consultancy; GSK: Consultancy. Kumar: Tenebio: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Novartis: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Bluebird Bio: Consultancy; Roche-Genentech: Consultancy, Research Funding; Merck: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Carsgen: Research Funding; Antengene: Consultancy, Honoraria; Oncopeptides: Consultancy; Beigene: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal